Cytotoxicity Test
1. Test Article Extraction: Cell culture medium
2. Test Cell Line: L929 cell line
3. Evaluation Items
(2) Qualitative Analysis: Cell morphology is assessed using Neutral Red staining
Skin Irritation Test
Control: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 6 animals
5. Route of Administration: Dermal patch contact
6. Dosing Frequency: Single application
7. Study Duration: Three days (excluding quarantine and acclimatization period)
8. Evaluation Items:
Intracutaneous Irritation Test
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 3 animals
5. Route of Administration: Intradermal injection
6. Dosing Frequency: Single administration
7. Study Duration: Three days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed prior to dosing and at 24, 48, and 72 hours post-dosing
Ocular Irritation Test
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 6 animals
5. Route of Administration: Instillation into the eye
6. Dosing Frequency: Single administration.
7. Study Duration: Three days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed prior to dosing and at 1, 24, 48, and 72 hours post-dosing
Oral Mucosa Irritation Test
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 12 animals
5. Route of Administration: Oral mucosa contact
6. Dosing Frequency: Repeated contact four times
7. Study Duration: Two days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed prior to dosing and at 1, 2, 3, 4 hours (hourly contact) and 24 hours post- dosing
(3) Tissue sectioning of the application site
(4) Histopathological evaluation
Vaginal Irritation Test
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 12 animals
5. Route of Administration: Vaginal mucosa contact
6. Dosing Frequency: Five applications (once daily for five days)
7. Study Duration: Six days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed prior to initial dosing, following each daily dosing, and at 24 hours after final dosing
(3) Tissue sectioning of the application site
(4) Histopathological evaluation
Penile Irritation Test
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 12 animals
5. Route of Administration: Penile contact
6. Dosing Frequency: Repeated contact four times
7. Study Duration: Three days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed prior to dosing and at 1, 24, and 48 hours after dosing
(3) Tissue sectioning of the application site
(4) Histopathological evaluation
Rectal Irritation Test
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 12 animals
5. Route of Administration: Rectal mucosa contact
6. Dosing Frequency: Five applications (once daily for five days)
7. Study Duration: Six days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed prior to initial dosing, following each daily dosing, and at 24 hours after final dosing
(3) Tissue sectioning of the application site
(4) Histopathological evaluation
Skin Sensitization Study (Guinea Pig Maximization Test)
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 30 animals
5. Route of Administration: Intradermal injection, Dermal patch application
6. Study Duration: Thirty days (excluding quarantine and acclimatization period)
7. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed during the test and at 24 and 48 hours after the end of the test
Acute Systemic Toxicity Test
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 20 animals
5. Route of Administration: Tail vein injection and intraperitoneal injection
6. Dosing Frequency: Single administration
7. Study Duration: Four days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Toxicity observation and scoring: Daily observation of appearance and behavior, and daily recording of body weight changes
Subacute Systemic Toxicity Test
1. Test Groups:
Control Group: Control article
3. Number of Animals: 20 animals (10 males, 10 females)
4. Route of Administration: (To be Professionally evaluated by Master Laboratory Co., Ltd.)
5. Dosing Frequency: (To be Professionally evaluated by Master Laboratory Co., Ltd.)
6. Study Duration: 24 hours to 28 days (To be Professionally evaluated by Master Laboratory Co., Ltd.)
7. Evaluation Items:
(2) Toxicity observation and scoring: Daily observation of appearance and behavior, weekly recording of food consumption and body weight changes, gross necropsy observations at termination
(3) Serum biochemical analysis
(4) Hematology
(5) Urinalysis
(6) Tissue sectioning
(7) Histopathological evaluation
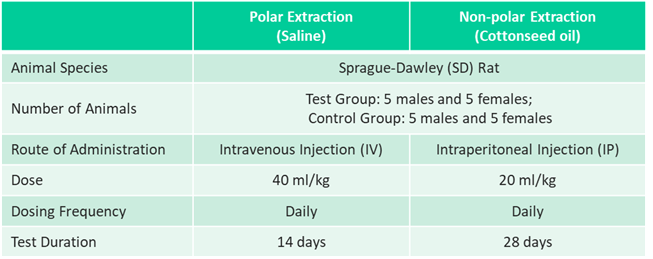
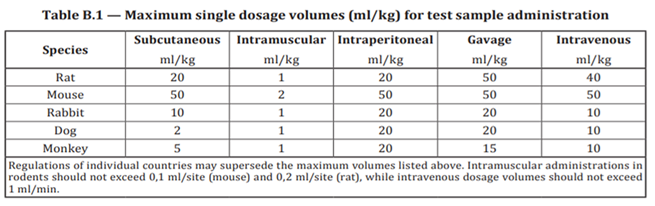
Chronic Systemic Toxicity Study
1. Test Groups:
Control Group: Control article
3. Number of Animals: 60 animals (30 males, 30 females)
4. Route of Administration: (To be professionally evaluated by Master Laboratory Co., Ltd.)
5. Dosing Frequency: (To be professionally evaluated by Master Laboratory Co., Ltd.)
6. Study Duration: 6-12 months (To be professionally evaluated by Master Laboratory Co., Ltd.)
7. Evaluation Items:
(2) Toxicity observation and scoring: Daily observation of appearance and behavior, weekly recording of food consumption and body weight changes, gross necropsy observations at termination
(3) Serum biochemical analysis
(4) Hematology
(5) Urinalysis
(6) Tissue sectioning
(7) Histopathological evaluation
Subchronic Systemic Toxicity Test
1. Test Groups:
Control Group: Control article
3. Number of Animals: 40 animals (20 males, 20 females)
4. Route of Administration: (To be Professionally evaluated by Master Laboratory Co., Ltd.)
5. Dosing Frequency: (To be professionally evaluated by Master Laboratory Co., Ltd.)
6. Study Duration: (To be professionally evaluated by Master Laboratory Co., Ltd.)
7. Evaluation Items:
(2) Toxicity observation and scoring: Daily observation of appearance and behavior, weekly recording of food consumption and body weight changes, gross necropsy observations at termination
(3) Serum biochemical analysis
(4) Hematology
(5) Urinalysis
(6) Tissue sectioning
(7) Histopathological evaluation
Subchronic Systemic Toxicity Test via Dual Routes of Parenteral Administration
1. Test Article Extraction: Two types of extracts (polar and non-polar)
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 24 animals (12 males, 12 females)
5. Route of Administration: Tail vein injection and intraperitoneal injection
6. Dosing Frequency:
Intraperitoneal 5 times (administered every three days)
8. Evaluation Items:
(2) Toxicity observation and scoring: Daily observation of appearance and behavior, weekly recording of food consumption and body weight changes, gross necropsy observations at termination
(3) Serum biochemical analysis
(4) Hematology
(5) Urinalysis
(6) Tissue sectioning
(7) Histopathological evaluation
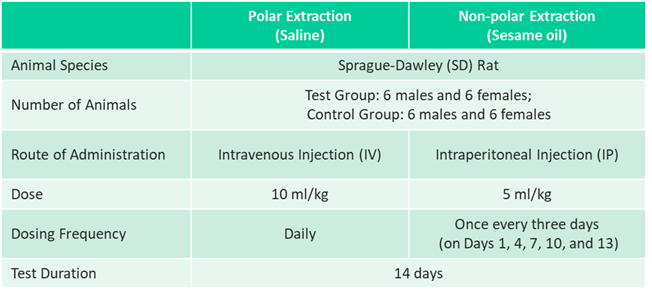
Pyrogen Test
1. Test Article Extraction: Single extract
2. Test Group: Test article extract (polar)
3. Test Animals: New Zealand White (NZW) Rabbits
4. Number of Animals: 3 animals
5. Route of Administration: Single administration
6. Dosing Frequency: Single administration
7. Study Duration: One day. (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Body temperature monitoring on the day of administration to evaluate the presence or absence of pyrogenic response
In Vivo Peripheral Blood Erythrocyte Micronucleus Test in Mice (MN Test)
OECD Guidelines for the Testing of Chemicals, Section 4: TG 471 — Bacterial Reverse Mutation Test
1. Test Article Extraction: Two types of extracts
2. Test System: CD-1 (ICR) mice
3. Test Groups: Negative Control Group, Positive Control Group, Two Extraction Solvent Control Groups, and Two Extract Groups
4. Test Conditions: Single or repeated dose administration via intraperitoneal or tail vein injection
5. Evaluation Item: Enumeration of micronucleated polychromatic erythrocytes (PCEs) or reticulocytes
In Vitro Mammalian Cell Gene Mutation Test Using the Thymidine Kinase Gene
OECD Guidelines for the Testing of Chemicals, Section 4: TG 490 — In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene
1. Test Article Extraction: Two types of extracts
2. Test System: L5178Y/TK+/- cells
3. Test Groups: Negative Control Group, Positive Control Group, Two Extraction Solvent Control Groups, and Two Extract Groups, performed in duplicates
4. Test Conditions:
Long-term treatment: without metabolic activation (S9 mix)
Bacterial Reverse Mutation Test (Ames Test)
1. Test Article Extraction: Two types of extracts
2. Test Strains: Salmonella typhimurium TA97a, TA98, TA100, TA102, TA1535
3. Test Groups: Negative Control Group, Positive Control Group, Two Extraction Solvent Control Groups, and Two Extract Groups, totaling 6 groups. Each group performed in triplicates
4. Test Conditions: Testing is performed with and without metabolic activation (S9 mix)
5. Evaluation Item: Analysis of the number of revertant colonies for each test group
Skin Sensitization Study (Guinea Pig Maximization Test)
1. Test Article Extraction: Two types of extracts (polar and non-polar)
2. Test Groups:
Control Group: Extraction solvent (polar), Extraction solvent (non-polar)
4. Number of Animals: 30 animals
5. Route of Administration: Intradermal injection, Dermal patch application
6. Study Duration: Thirty days (excluding quarantine and acclimatization period)
7. Evaluation Items:
(2) Irritation observation and scoring: Observation and scoring performed during the test and at 24 and 48 hours after the end of the test
Subcutaneous Implantation Test
1. Test Article Implantation: Size conforming to the standards. (To be professionally evaluated by Master Laboratory Co., Ltd.)
2. Test Groups:
Control Group: Negative control
4. Number of Animals: 3 animals
5. Route of Administration: Surgical subcutaneous implantation
6. Dosing Frequency: Single administration
7. Study Duration: (To be professionally evaluated by Master Laboratory Co., Ltd.)
8. Evaluation Items:
(2) Daily observation of animal status post-surgery, animal body weight measurement during the test period
(3) Gross necropsy observations at termination
(4) Tissue sectioning
(5) Histopathological evaluation
Muscle Implantation Test
1. Test Article Implantation: Size conforming to the standards. (To be professionally evaluated by Master Laboratory Co., Ltd.)
2. Test Groups:
Control Group: Negative control
4. Number of Animals: 3 animals
5. Route of Administration: Surgical intramuscular implantation
6. Dosing Frequency: Single administration
7. Study Duration: (To be professionally evaluated by Master Laboratory Co., Ltd.)
8. Evaluation Items:
(2) Daily observation of animal status post-surgery, animal body weight measurement during the test period
(3) Gross necropsy observation after sacrifice
(4) Tissue sectioning
(5) Histopathological evaluation
Bone Implantation Test
1. Test Article Implantation: Size conforming to the standards. (To be professionally evaluated by Master Laboratory Co., Ltd.)
2. Test Groups:
Control Group: Negative control
4. Number of Animals: 5 animals
5. Route of Administration: Surgical Implantation in the Femur
6. Dosing Frequency: Single administration
7. Study Duration: (To be professionally evaluated by Master Laboratory Co., Ltd.)
8. Evaluation Items:
(2) Daily observation of animal status post-surgery, animal body weight measurement during the test period
(3) Gross necropsy observation after sacrifice
(4) Tissue sectioning
(5) Histopathological evaluation
22-Day Ocular Irritation Test (Rabbit Contact Lens Wear Test)
1. Test Article: Direct contact lens wear
2. Test Groups:
Control Group: Control Article
4. Number of Animals: 6 animals
5. Route of Administration: Ocular contact
6. Dosing Frequency: Daily.
7. Study Duration: Twenty-two days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Daily observation of animal status and test article condition, periodic Fluorescein staining and slit-lamp biomicroscopy
(3) Tissue sectioning of the application site
(4) Histopathological evaluation
Repeated Dose 28-day Oral Toxicity Study
1. Test Article Dosage: (To be professionally evaluated by Master Laboratory Co., Ltd.)
2. Test Groups:
Test Group: Mid dose
Test Group: High dose
Control Group: Negative control
4. Number of Animals: 80 animals (40 males, 40 females)
5. Route of Administration: Oral gavage
6. Dosing Frequency: Daily
7. Study Duration: 28 days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Toxicity observation and scoring: Daily observation of appearance and behavior, weekly recording of food consumption and body weight changes, gross necropsy observation after sacrifice
(3) Serum biochemical analysis (Clinical Chemistry)
(4) Hematology
(5) Urinalysis
(6) Tissue sectioning
(7) Histopathological evaluation
Acute Oral Toxicity Study (Limit Test)
1. Test Article Dosage: (To be professionally evaluated by Master Laboratory Co., Ltd.)
2. Test Groups:
Control Group
4. Number of Animals: 20 animals (10 males, 10 females)
5. Route of Administration: Oral gavage
6. Dosing Frequency: Single administration
7. Study Duration: 14 days (excluding quarantine and acclimatization period)
8. Evaluation Items:
(2) Toxicity observation and scoring: Daily observation of appearance and behavior, weekly recording of food consumption and body weight changes, gross necropsy observation after sacrifice
