ISO 10993-1 and the latest FDA guidelines emphasize a risk-based approach to biocompatibility evaluation, aimed at ensuring the safety and effectiveness of medical devices. These standards focus not only on basic testing but also require manufacturers to have a comprehensive understanding of the materials used in their devices and their clinical applications. The goal is to manage and minimize the likelihood and severity of potential risks through appropriate testing methods.
Master Laboratory Co., Ltd. has established a professional laboratory accredited by the Taiwan Accreditation Foundation (TAF) and compliant with the OECD Principles of Good Laboratory Practice, providing comprehensive support to clients in identifying and managing potential biocompatibility risks.
Our team comprises professional veterinarians, a dedicated pathology group, and experienced pathologists, supported by state-of-the-art testing equipment. With this strong foundation, we deliver comprehensive biocompatibility and performance testing for high-risk medical devices. Moreover, in collaboration with expert consultants, we provide customized test designs and professional review services to support the diverse needs of medical device development.
To date, we have successfully supported clients in Taiwan, the United Kingdom, South Korea, and China in obtaining regulatory approvals and certifications. Our biocompatibility and performance testing reports have played a key role in helping clients register their medical devices in major markets, including Taiwan, the EU, the U.S., South Korea, and China.
Looking ahead, we will continue to expand our expertise in medical device safety certification, striving to bring high-quality medical devices to the global market through the dedication and professionalism of Master Laboratory Co., Ltd.
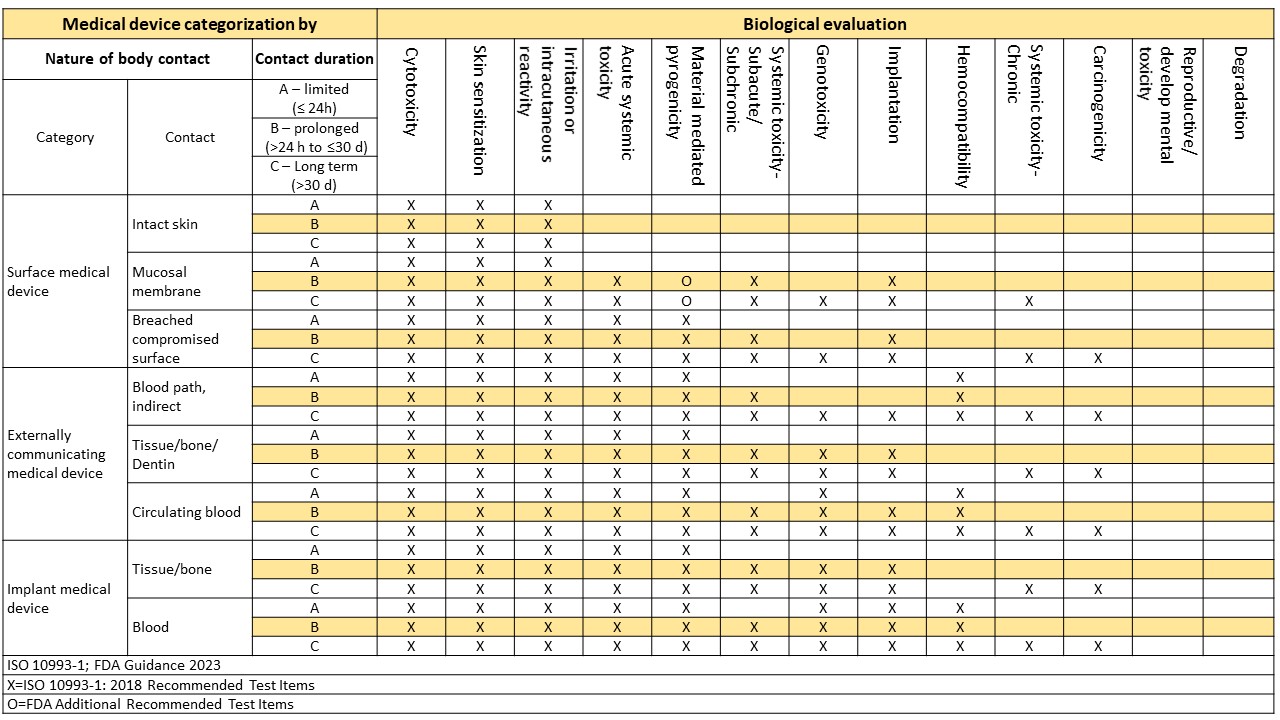
Cytotoxicity Test
1. Cytotoxicity Test: ISO 10993-5, USP <87>
Irritation Test
1. Skin Irritation Test: ISO 10993-23
2. Intracutaneous Irritation Test: ISO 10993-23
3. Ocular Irritation Test: ISO 10993-23
4. Oral Mucosa Irritation Test: ISO 10993-23
5. Vaginal Irritation Test: ISO 10993-23
6. Penile Irritation Test: ISO 10993-23
7. Rectal Irritation Test: ISO 10993-23
8. Intracutaneous Irritation Test for USP <88> Class VI Plastic Materials: USP <88>
Skin Sensitization Test
1. Sensitization Test (Guinea Pig Maximization Test, GPMT): ISO 10993-10
2. Sensitization Test (Buehler Test): ISO 10993-10
Systemic Toxicity Test
1. Acute Systemic Toxicity Test: ISO 10993-11
2. Subacute Systemic Toxicity Test: ISO 10993-11
3. Chronic Systemic Toxicity Test: ISO 10993-11
4. Subchronic Systemic Toxicity Test: ISO 10993-11
5. Subchronic Systemic Toxicity Test via Dual Routes of Parenteral Administration: ISO 10993-11
6. Acute Systemic Toxicity Test for USP <88> Class VI Plastic Materials: USP <88>
Pyrogen Test
1. Pyrogen Test: USP <151>
Genotoxicity Test
1. In Vivo Peripheral Blood Erythrocyte Micronucleus Test in Mice (MN Test): ISO 10993-3
2. In Vitro Mammalian Cell Gene Mutation Test Using the Thymidine Kinase Gene: ISO 10993-3
3. Bacterial Reverse Mutation Test (Ames Test): ISO 10993-3, OECD 471
Implantation Test
1. Subcutaneous Implantation Test: ISO 10993-6
2. Muscle Implantation Test: ISO 10993-6
3. Bone Implantation Test: ISO 10993-6
4. Brain Implantation Test: ISO 10993-6
5. Muscle Implantation Test for USP <88> Class VI Plastic Materials: USP <88>
Hemocompatibility Test
In Vivo Degradation Test
Contact Us
